今日,医药行业传来一条重磅新闻——辉瑞(Pfizer)宣布,美国FDA加速批准了其新药BESPONSA(inotuzumab ozogamicin)上市,治疗成人复发或难治性B细胞前体急性淋巴性白血病(B-cell precursor acute lymphoblastic leukemia)。值得一提的是,这是首款获得美国FDA批准的靶向CD22的抗体药物偶联物(ADC)。
急性淋巴性白血病是一种侵袭性极高的白血病,在成人患者中的预后极差。据估计,2017年全美大约有5970个新发病例,其中大约40%为成人患者。目前,治疗这种疾病的标准疗法是长期的高强度化疗。在初始治疗后,有10%-20%的患者的病情无法得到有效缓解,这意味着他们对治疗没有产生响应。此外,在那些出现缓解的患者中,又有一半患者的病情会出现复发。据估计,疾病复发后的中位数生存期,仅为4.5个月到6个月。因此,这些患者急需创新的治疗手段。
由辉瑞与其合作伙伴联合带来的BESPONSA就是这样一款充满潜力的新疗法。它是一款创新的抗体药物偶联物,由两部分组成。其中一部分是靶向CD22的单克隆抗体,另一部分是细胞毒剂卡奇霉素(calicheamicin)。它的作用机理很容易理解:CD22抗原在B细胞表面普遍存在,因此这款ADC能够靶向癌细胞,并与之表面的CD22抗原结合。随后,这些ADC会被内吞入癌细胞。随后,卡奇霉素会发挥它的功效,造成癌细胞的死亡。先前,它曾获得美国FDA颁发的突破性疗法认定。
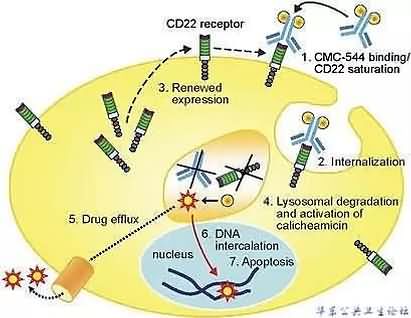
▲Inotuzumab ozogamicin(CMC-544)的作用机理(图片来源:《Leukemia》)
在一项随机、公开标签、多中心的国际临床试验中,BESPONSA的安全性与疗效得到了评估。这项研究一共招募了326名成人患者。他们其中的一部分接受BESPONSA治疗,另一部分则接受化疗。研究发现,BESPONSA组中,患者的完全缓解率(CR)为81%(95% CI:72%-88%),而化疗组的数据仅为29%(95% CI:21%-39%)。在取得完全缓解的所有患者中,接受BESPONSA治疗的患者其最小病灶残留(MRD)的阴性率也更高,为78%(95% CI:68%-87%),化疗组的这一数字是28%(95% CI:14%-47%)。此外,BESPONSA组患者的中位数总生存期为7.7个月(95% CI:6.0个月-9.2个月),化疗组的数据为6.2个月(95% CI:4.7个月-8.3个月)。在安全性上,研究人员指出这款新药可能会带来肝毒性。基于BESPONSA在临床上为患者带来的改善,美国FDA在其申请上市后曾授予它优先审评资格,并于今日加速批准这款新药上市。
“BESPONSA的批准对于复发性或难治性B细胞急性淋巴性白血病的成人患者来说,是重要的一步。这是一种致命的罕见疾病,如果不治疗,会在短短几个月里夺去人的生命,”辉瑞肿瘤部的全球总裁Liz Barrett女士说道:“BESPONSA带来了新疗法,帮助解决了重大的需求,也能让更多的患者坚持到进行干细胞移植的那一刻。想要取得长期缓解,这是最好的手段。能够践行对恶性血液癌症患者的承诺,我们感到非常自豪。我们也将继续为急性淋巴性白血病和其他血液癌症找到新的治疗方法。”
我们祝贺辉瑞的这款创新抗体药物偶联物顺利上市,并祝愿它能为患者的生活带来重要改变,挽救更多生命。
参考资料:
[1] Pfizer Receives U.S. FDA Approval for BESPONSA® (inotuzumab ozogamicin)
[2] Pfizer’s CD22-targeted cancer drug inotuzumab wins an accelerated OK at FDA
[3] FDA approves new treatment for adults with relapsed or refractory acute lymphoblastic leukemia
辉瑞 Prizer 链接
PFIZER RECEIVES U.S. FDA APPROVAL FOR BESPONSA® (INOTUZUMAB OZOGAMICIN)
BESPONSA is the first and only CD22-directed antibody-drug conjugate indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia
Pfizer Inc. (NYSE:PFE) today announced that the U.S. Food and Drug Administration (FDA) has approved BESPONSA® (inotuzumab ozogamicin) for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).1 BESPONSA was reviewed and approved under the FDA’s Breakthrough Therapy designation and Priority Review programs.
“The approval of BESPONSA is an important step forward for adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia, a rare disease that can be fatal within a matter of months if left untreated,” said Liz Barrett, global president, Pfizer Oncology. “BESPONSA will help address a significant need for new treatment options in B-cell acute lymphoblastic leukemia, and may help more patients reach stem cell transplant, which provides the best chance for long term remission. We’re proud to build on our continued commitment to patients with hematologic malignancies, and will continue our work to find new treatments in acute lymphoblastic leukemia and other blood cancers.”
The approval was based on results from the Phase 3 INO-VATE ALL trial, a randomized, open-label, international, multicenter study evaluating the safety and efficacy of BESPONSA compared with Investigator’s choice of chemotherapy in 326 adult patients with relapsed or refractory B-cell ALL.1
“Based on the results seen in the INO-VATE ALL trial, BESPONSA improved multiple efficacy measures, including rates of hematologic remission, MRD-negativity and stem cell transplantation,” said Hagop M. Kantarjian, M. D., INO-VATE ALL lead study investigator and professor, The University of Texas MD Anderson Cancer Center. “I look forward to seeing the impact this important new therapy may have on my patients.”
The complete remission rate (CR/CRi)* for patients treated with BESPONSA was 81 percent [95% CI: 72%-88%] compared to 29 percent with chemotherapy [95% CI: 21%-39%]. Among patients achieving CR/CRi, those treated with BESPONSA also demonstrated a higher rate of minimal residual disease (MRD) negativity (78% [95% CI: 68%-87%]) compared to those treated with chemotherapy (28% [95% CI: 14%-47%]). Forty-eight percent of patients treated with BESPONSA proceeded to hematopoietic stem cell transplantation (HSCT) compared to 22 percent treated with chemotherapy. The median overall survival (OS) for patients treated with BESPONSA was 7.7 months [95% CI: 6.0, 9.2] and 6.2 months [95% CI: 4.7, 8.3] for patients treated with chemotherapy. The analysis of OS for patients treated with BESPONSA compared to chemotherapy did not meet the pre-specified boundary for statistical significance (HR: 0.75 [97.5% CI: 0.57-0.99]).1
The U.S. labeling for BESPONSA includes a boxed warning for hepatotoxicity, including hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), and increased risk of post-HSCT non-relapse mortality. Veno-occlusive disease, including fatal and life-threating VOD, occurred in 14 percent of patients treated with BESPONSA. A higher post-HSCT non-relapse mortality rate occurred in patients treated with BESPONSA (39%) than chemotherapy (23%).1 In patients treated with BESPONSA, the most common (≥20%) adverse reactions were thrombocytopenia, neutropenia, infection, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, gamma-glutamyltransferase increased, and hyperbilirubinemia.1
Pfizer is committed to helping patients gain access to Pfizer medicines, including BESPONSA, and related educational tools, resources and services, regardless of their financial or health insurance status through the company’s patient assistance programs. Patients can call 1-877-744-5675 to learn more.
The full Prescribing Information, including BOXED WARNING, for BESPONSA can be found athttps://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf.
IMPORTANT BESPONSA® (inotuzumab ozogamicin) SAFETY INFORMATION FROM THE U.S. PRESCRIBING INFORMATION
WARNING: HEPATOTOXICITY, INCLUDING HEPATIC VENO-OCCLUSIVE DISEASE (VOD) (ALSO KNOWN AS SINUSOIDAL OBSTRUCTION SYNDROME) and INCREASED RISK OF POST–HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT) NON-RELAPSE MORTALITY (NRM):
- Hepatotoxicity, including fatal and life-threatening VOD, occurred in patients who received BESPONSA. The risk of VOD was greater in patients who underwent HSCT after BESPONSA treatment. Consider identified risk factors. Monitor closely for signs and symptoms of VOD
- There was a higher post-HSCT non-relapse mortality rate in patients receiving BESPONSA, resulting in a higher Day 100 post-HSCT mortality rate
Hepatotoxicity, Including VOD:
Hepatotoxicity, including fatal and life-threatening VOD, occurred in patients who received BESPONSA. The risk of VOD was greater in patients who underwent HSCT after BESPONSA treatment. The use of HSCT conditioning regimens containing 2 alkylating agents and last total bilirubin ≥ the upper limit of normal (ULN) before HSCT were significantly associated with an increased risk of VOD. Other risk factors for VOD in patients treated with BESPONSA included ongoing or prior liver disease, prior HSCT, increased age, later salvage lines, and a greater number of BESPONSA treatment cycles. Grade 3/4 increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin have occurred.
Monitor closely for signs and symptoms of VOD; these may include elevations in total bilirubin, hepatomegaly (which may be painful), rapid weight gain, and ascites. Elevation of liver tests may require dosing interruption, dose reduction, or permanent discontinuation of BESPONSA. Permanently discontinue treatment if VOD occurs. If severe VOD occurs, treat according to standard medical practice.
Increased Risk of Post-HSCT Non-Relapse Mortality: There was a higher post-HSCT non-relapse mortality rate in patients receiving BESPONSA, resulting in a higher Day 100 post-HSCT mortality rate. In the BESPONSA arm, the most common causes of post-HSCT non-relapse mortality included VOD and infections. Monitor closely for toxicities post HSCT, including signs and symptoms of infection and VOD.
Myelosuppression: Myelosuppression, and severe, life-threatening and fatal complications of myelosuppression, including hemorrhagic events and infections, have occurred with BESPONSA. Thrombocytopenia, neutropenia, and febrile neutropenia were reported.
Monitor complete blood counts prior to each dose of BESPONSA and monitor for signs and symptoms of infection, bleeding/hemorrhage, or other effects of myelosuppression during treatment and provide appropriate management. As appropriate, administer prophylactic anti-infectives during and after treatment with BESPONSA. Dose interruption, dose reduction, or permanent discontinuation may be required.
Infusion-Related Reactions: Infusion-related reactions have occurred in patients who received BESPONSA. Premedicate with a corticosteroid, antipyretic, and antihistamine prior to dosing. Monitor patients closely during and for at least 1 hour after the end of the infusion for the potential onset of infusion-related reactions. Interrupt the infusion and institute appropriate medical management if an infusion-related reaction occurs. For severe or life-threatening infusion reactions, permanently discontinue BESPONSA.
QT Interval Prolongation: Increases in QT interval have occurred. Administer BESPONSA with caution in patients who have a history of or predisposition to QTc prolongation, who are taking medicinal products that are known to prolong QT interval, and in patients with electrolyte disturbances. Obtain electrocardiograms and electrolytes prior to treatment and after initiation of any drug known to prolong QTc, and periodically monitor as clinically indicated during treatment.
Embryo-Fetal Toxicity and Nursing Mothers: BESPONSA can cause embryo-fetal harm. Advise males and females of reproductive potential to use effective contraception during BESPONSA treatment and for at least 5 and 8 months after the last dose, respectively. Advise women against breastfeeding while receiving BESPONSA and for 2 months after the last dose.
Adverse Reactions: The most common (≥20%) adverse reactions observed with BESPONSA were thrombocytopenia, neutropenia, infection, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, gamma-glutamyltransferase increased, and hyperbilirubinemia.
The most common (≥2%) serious adverse reactions were infection, febrile neutropenia, hemorrhage, abdominal pain, pyrexia, VOD, and fatigue.
Please see full Prescribing Information, including BOXED WARNING herehttps://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761040s000lbl.pdf.
About Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is an aggressive type of leukemia with a poor prognosis in adults.2 The current foundational treatment is intensive, long-term chemotherapy.3 In 2017, it is estimated that 5,970 cases of ALL will be diagnosed in the United States.4 About 4 in 10 cases occur in adults.4 While about 80-90% of adult patients will have a complete remission at some point during initial treatment, the remainder (approximately 10%-20%) will be refractory, meaning they no longer respond to treatment.3 Additionally, about half of patients who achieve remission will relapse.3 The post relapse median survival is 4.5 to 6 months.5
About BESPONSA® (inotuzumab ozogamicin)
BESPONSA is an antibody-drug conjugate (ADC) composed of a monoclonal antibody (mAb) targeting CD22, a cell surface antigen expressed on cancer cells in almost all B-ALL patients, linked to a cytotoxic agent.6 When BESPONSA binds to the CD22 antigen on B-cells, it is internalized into the cell, where the cytotoxic agent calicheamicin is released causing cell death.7 BESPONSA is administered as a one-hour intravenous infusion that can be given in the outpatient setting of care for appropriate patients.
BESPONSA originates from a collaboration between Pfizer and Celltech, now UCB. Under the terms of this agreement, Pfizer has sole responsibility for all commercialization, manufacturing and clinical development activities for this molecule. Pfizer also collaborated with SFJ Pharmaceuticals Group on the registrational program (INO-VATE ALL) for BESPONSA.
About Pfizer Oncology
Pfizer Oncology is committed to pursuing innovative treatments that have a meaningful impact on those living with cancer. As a leader in oncology speeding cures and accessible breakthrough medicines to patients, Pfizer Oncology is helping to redefine life with cancer. Our strong pipeline of biologics, small molecules and immunotherapies, one of the most robust in the industry, is studied with precise focus on identifying and translating the best scientific breakthroughs into clinical application for patients across a wide range of cancers. By working collaboratively with academic institutions, individual researchers, cooperative research groups, governments and licensing partners, Pfizer Oncology strives to cure or control cancer with its breakthrough medicines. Because Pfizer Oncology knows that success in oncology is not measured solely by the medicines you manufacture, but rather by the meaningful partnerships you make to have a more positive impact on people’s lives.
Pfizer Inc.: Working together for a healthier worldTM
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products. Our global portfolio includes medicines and vaccines as well as many of the world's best-known consumer health care products. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world's premier innovative biopharmaceutical companies, we collaborate with health care providers, governments and local communities to support and expand access to reliable, affordable health care around the world. For more than 150 years, we have worked to make a difference for all who rely on us. We routinely post information that may be important to investors on our website at www.pfizer.com. In addition, to learn more, please visit us on www.pfizer.com and follow us on Twitter at @Pfizer and @Pfizer_News, LinkedIn, YouTube and like us on Facebook at Facebook.com/Pfizer.
DISCLOSURE NOTICE: The information contained in this release is as of August 17, 2017. Pfizer assumes no obligation to update forward-looking statements contained in this release as the result of new information or future events or developments.
This release contains forward-looking information about BESPONSA (inotuzumab ozogamicin), and an approval by the FDA for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia, including its potential benefits, that involves substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Risks and uncertainties include, among other things, uncertainties regarding the commercial success of BESPONSA; the uncertainties inherent in research and development, including the ability to meet anticipated clinical trial commencement and completion dates and regulatory submission dates, as well as the possibility of unfavorable clinical trial results, including unfavorable new clinical data and additional analyses of existing clinical data; whether and when applications for BESPONSA may be filed in any other jurisdictions; whether and when any such applications that may be pending or filed for BESPONSA may be approved by regulatory authorities, which will depend on the assessment by such regulatory authorities of the benefit-risk profile suggested by the totality of the efficacy and safety information submitted; decisions by regulatory authorities regarding labeling and other matters that could affect the availability or commercial potential of BESPONSA; and competitive developments.
A further description of risks and uncertainties can be found in Pfizer’s Annual Report on Form 10-K for the fiscal year ended December 31, 2016 and in its subsequent reports on Form 10-Q, including in the sections thereof captioned “Risk Factors” and “Forward-Looking Information and Factors That May Affect Future Results”, as well as in its subsequent reports on Form 8-K, all of which are filed with the U.S. Securities and Exchange Commission and available at www.sec.gov and www.pfizer.com.
*CR/CRi includes complete remission (<5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, full recovery of peripheral blood counts and resolution of any extramedullary disease) and complete remission with incomplete recovery of peripheral blood counts.
1 BESPONSA (inotuzumab ozogamicin) Prescribing Information. New York. NY: Pfizer Inc: 2017.
2 National Cancer Institute: Adult Acute Lymphoblastic Leukemia Treatment (PDQ®) – General Information About Adult Acute Lymphoblastic Leukemia (ALL). Available at: https://www.cancer.gov/types/leukemia/hp/adult-all-treatment-pdq#section.... Accessed March 23, 2017.
3 American Cancer Society: Typical treatment of acute lymphocytic leukemia. Available at: https://www.cancer.org/cancer/acute-lymphocytic-leukemia/treating/typica.... Accessed March 21, 2017.
4 American Cancer Society: What are the key statistics about acute lymphocytic leukemia? Available at:http://www.cancer.org/cancer/leukemia-acutelymphocyticallinadults/detail.... Accessed March 21, 2017.
5 National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Acute Lymphoblastic Leukemia. Version 2.2016. National Comprehensive Cancer Network; 2016. http://www.nccn.org/professionals/physician_gls/pdf/all.pdf. Accessed April 26, 2017.
6 Leonard J et al. Epratuzumab, a Humanized Anti-CD22 Antibody, in Aggressive Non-Hodgkin’s Lymphoma: a Phase I/II Clinical Trial Results. Clinical Cancer Research. 2004; 10: 5327-5334.
7 DiJoseph JF. Antitumor Efficacy of a Combination of CMC-544 (Inotuzumab Ozogamicin), a CD22-Targeted Cytotoxic Immunoconjugate of Calicheamicin, and Rituximab against Non-Hodgkin’s B-Cell Lymphoma. Clin Cancer Res. 2006; 12: 242-250.
Media:
Sally Beatty, 212-733-6566
or
Investor:
Ryan Crowe, 212-733-8160